Research group led by Prof. Ping Chen from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences was recently invited to publish a review article on Cell Reports Physical Science, titled “Hydrides Mediate Nitrogen Fixation”. Prof. Chen's group has long been devoted to the research of hydrogen-mediated activation and fixation of nitrogen molecules. (Nature Chemistry, 2017; Nature Energy, 2018; Nature Catalysis, 2021).
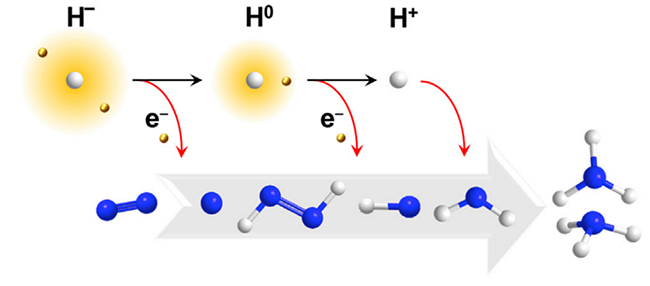
The fixation of atmospheric dinitrogen to ammonia is crucial for the sustainable growth of human society. The reduction of N2 to NH3 needs the inputs of electrons(e-) and protons (H+) which could be supplied by hydridic hydrogens, while hydride, as e-/H+ carrier, can participate in the nitrogen reduction reaction through the transformation from hydridic hydrogen (H-) to neutral hydrogen (H0) and further to protonic hydrogen (H+)(as shown in the figure). In this review, the author discuss representative progresses on the exploration of molecular and bulk phase hydrides for dinitrogen activation and reduction with a focus on the active involvement and function mechanism of reactive hydride compounds in facilitating ammonia production under mild conditions. They conclude that, with the versatile and important roles discovered, hydrides have the power in mediating electron and/or hydrogen transfers for N2 fixation via energetically more accessible pathways, which in turn would stimulate new ideas for the design and development of more efficient catalysts or processes for green ammonia production.
This work was supported by National Natural Science Foundation of China and the Youth Innovation Promotion Association of Chinese Academy of Sciences. (Text Yeqin Guan).
Article link:https://doi.org/10.1016/j.xcrp.2022.100779

