
Research
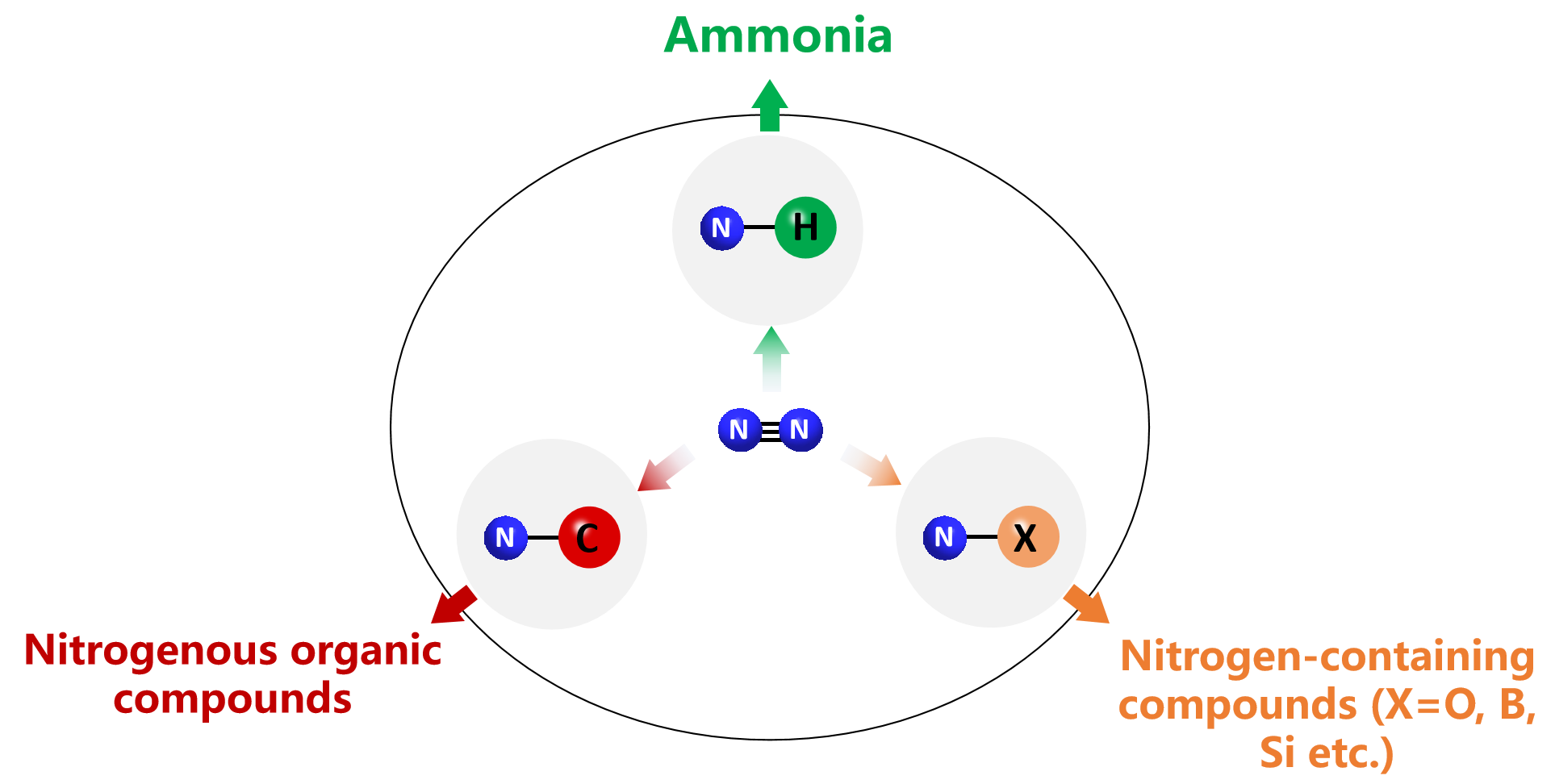
I. N2 fixation to NH3 as well as other nitrogen-containing compounds
Motivation:
Nitrogen atoms are essential component of fertilizers and medicaments. The fixation of the stable dinitrogen molecules under mild conditions has been a long-sought-after goal in chemistry considering the critical need to reduce the energy consumption and carbon dioxide emission.
Our work:
Ø To develop metal hydrides as efficient catalysts for N2 fixation under mild conditions;
Ø To study the reaction mechanism of N2 reduction and hydrogenation by metal hydrides;
Ø To study the photochemical and electrochemical properties of metal hydrides for more efficient N2 fixation processes driven by renewable energy.
Representative publications:
1. Breaking scaling relations to achieve low-temperature ammonia synthesis through LiH-mediated nitrogen transfer and hydrogenation, Nature Chemistry, 9(1): 64-70, 2017.
Paper link: https://www.nature.com/articles/nchem.2595
The cooperation of transition metal (TM) and LiH can break the TM-exerted scaling relations and creates an energy-efficient pathway that allows NH3 synthesis under mild conditions.
2. Production of ammonia via a chemical looping process based on metal imides as nitrogen carriers, Nature Energy, 3(12): 1067-1075, 2018.
Paper link: https://www.nature.com/articles/s41560-018-0268-z
Alkali and alkaline earth metal imides can function as nitrogen carriers that mediate ammonia production via a two-step chemical looping process operating under mild conditions.
3. Ternary ruthenium complex hydrides for ammonia synthesis via the associative mechanism, Nature Catalysis, 4: 959–967, 2021.
Paper link: https://www.nature.com/articles/s41929-021-00698-8
The ternary ruthenium complex hydrides Li4RuH6 and Ba2RuH6, facilitates an associative reaction mechanism for ammonia production from N2 + H2 with superior kinetics under mild conditions.
4. Light-driven ammonia synthesis under mild conditions using lithium hydride, Nature Chemistry, 16, 373-379, 2024.
Paper link: https://www.nature.com/articles/s41557-023-01395-8
Lithium hydride is shown to mediate photo-driven N2 fixation under ambient conditions with a fundamentally different reaction mechanism.
II. Hydrides-mediated transformation of small organic molecules such as alkynes, alkenes, and alkanes
Motivation: The hydrogenation/dehydrogenation, hydrogenolysis, or reforming of alkynes, alkenes, and alkanes are important catalytic processes.
Our work: We are aiming to develop novel and advanced hydride materials for the transformation of these key molecules with high activity and selectivity.
Representative publications:
1. Transition Metal-Free Hydrogenolysis of Anilines to Arenes Mediated by Lithium Hydride, Journal of the American Chemical Society, 144(38): 17441-17448, 2022.
Paper link: https://pubs.acs.org/doi/10.1021/jacs.2c05586?ref=pdf
Lithium hydride (LiH) enables the hydrogenolysis of aniline to benzene and ammonia via a unique pathway in which Li-mediated nucleophilic attack of hydride to the α-sp2 C atom of aniline plays an important role.
2. Enabling semihydrogenation of alkynes to alkenes by using a calcium palladium complex hydride, Journal of the American Chemical Society, 143(49): 20891-20897, 2021.
Paper link: https://pubs.acs.org/doi/full/10.1021/jacs.1c09489
The complex palladium hydride such as CaPdH2 can enable selective hydrogenation of alkynes to alkenes.