
Research
Objectives:
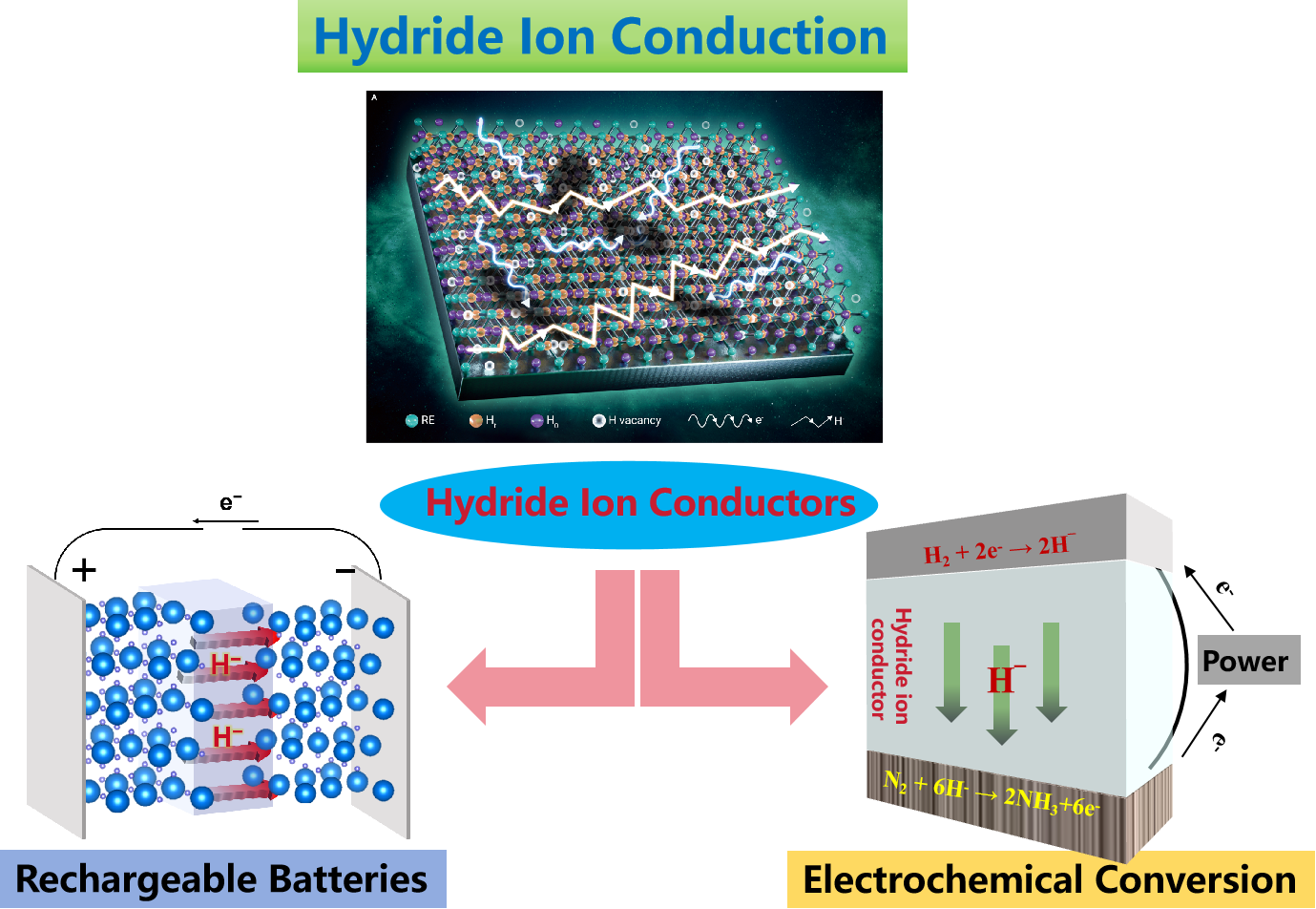
Development of Hydride Ion Conductors
• Conductor Synthesis: To develop a series of hydride ion conductors capable of operating across various temperature ranges and meeting the demands of diverse application scenarios.
• Performance Improvement: To develop new hydride ion conductors with high ionic conductivity, negligible electronic conductivity, and high thermal and electrochemical stability.
• Mechanism Study: To study the relationship between compositions, structures, and properties of hydride ion conductors.
Rechargeable Hydride Ion Batteries
• Battery System Exploration: To identify and explore novel electrode materials for use in hydride ion batteries.
• Battery Manufacturing Improvement: To develop new manufacturing methods for hydride ion batteries, aiming to achieve high capacities and excellent cyclic stability.
Electrochemical Conversion Reactions
• To develop new electrochemical conversion systems based on hydride ion conduction, focusing on exploring hydrogen-involved reactions under mild conditions.
Why?
• Development of Hydride Ion Conductors: Current limitations such as low ionic conductivity, significant electronic conductivity, poor thermal and electrochemical stability, and insufficient material diversity hinder the practical application of hydride ion conductors.
• Rechargeable Hydride Ion Batteries: Lithium-ion batteries face challenges like dendrite formation and resource scarcity, which can be mitigated by hydride ion batteries.
• Electrochemical Conversion Reactions: Many hydrogen-involved reactions require harsh conditions and consume substantial energy. Hydride ions, composed of one hydrogen atom and two electrons, offer unique advantages in these reactions, particularly in reducing N2 and CO2 under milder conditions.
Challenges:
• Development of Hydride Ion Conductors: Achieving simultaneous fast hydride ion diffusion, negligible electron conduction, high thermal and electrochemical stability, and compatibility with electrodes is challenging.
• Rechargeable Hydride Ion Batteries: Exploring new electrode materials with high capacities, electrolyte compatibility, and good reversibility, along with developing innovative manufacturing methods, remains a critical challenge.
• Electrochemical Conversion Reactions: Constructing new electrochemical reaction systems that enable various hydrogen-involved reactions under mild conditions is difficult.
Vision:
• Development of Hydride Ion Conductors: To create new hydride ion conductors with outstanding overall performance, suitable for both battery applications and electrochemical reactions.
• Rechargeable Hydride Ion Batteries: To pioneer a new class of battery systems fundamentally different from current technologies, addressing safety and resource issues.
• Electrochemical Conversion Reactions: To realize hydrogen-involved reactions under mild conditions using hydride-ion-conducting systems.
Papers
• Nature, 2025, 646, 338–342.
• Nature, 2023, 616, 73–76.
• Angewandte Chemie International Edition, 2024, e202417610.
• Journal of Energy Chemistry, 2024, 95, 40–445.
Reviews and perspectives
• The Innovation Materials, 2023, 1, 100006.
• Trends in Chemistry, 2022, 4, 935-947.