Recently, a team led by Professor Chen Ping, Associate Professor Guo Jianping, and Dr. Chang Fei made new advancements in synthetic ammonia research using manganese-based catalysts. The relevant research findings were fully published in the Journal of the American Chemical Society (J. Am. Chem. Soc., DOI: 10.1021/jacs.8b08334).
The synthesis of ammonia on transition metals is an important research topic in the field of heterogeneous catalysis.RuandFe haveexcellent catalytic performance in the synthesis of ammonia dueto their moderateN adsorption energy, and are used in the industrial synthesis of ammonia. V, Cr, Mn,and other early transition metals have strong adsorption ofNspecies and form stable nitride phases in the reaction atmosphere of ammonia synthesis, which hinders the subsequent hydrogenation step and has poor activity. Therefore, they have not attracted widespread attention fromresearchers for a long time.
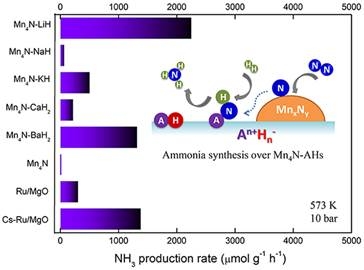
To address this issue, the research team, based on previous work (Nature Chem.,ACS Catal..,Angew. Chem. Int. Ed.), selectedMnmetal as a representative and systematically investigated the effects of alkaline (earth) metal hydrides (LiH, NaH, KH, BaH2, CaH2) on the catalyticactivityofMnmetal in synthesizing ammonia. The experimental results show that the addition of alkaline (earth) metal hydrides can increase the catalytic activity ofMnin synthesizing ammoniaby 1-3orders of magnitude, among whichMn-LiHandMn-BaH2can even be comparable to precious metalRu-based catalysts. In addition, the research results also show that the order ofpromoting effect of alkaline (earth) metal hydrides onMnis significantly different from that ofconventional oxide or hydroxide promoters. Thermodynamic analysis and characterization results reveal that under the conditions of ammonia synthesis reaction, the phase transition between alkaline (earth) metal hydrides and imino compounds, and their interaction with manganese nitride,are the essential reasons for their promoting effect. In-depthinvesuigationon the structure-activity relationship ofthe Mn-LiHcatalytic system found that the active phase of the catalyst and its kinetic behavior (apparent activation energy, rate-controlling step, etc.) strongly depend on reaction conditions such as temperature and space velocity, which is another feature that distinguishes it from conventional ammonia synthesis catalysts. This research resultsmay provide an effective catalyst design strategy for "activating" the catalytic behavior of early transition metals in ammonia synthesis.
This research was completed in collaboration with Professor Li XingguofromPeking University. Thestudywas funded by the National Natural Science Foundation of China, the China-Japan Intergovernmental Cooperation Project, the Collaborative Innovation Center for Energy Materials Chemistry of the Ministry of Education (iChEM), the Basic Research Project of Methanol Conversion and Coal-to-Oil New Technologies of our Institute (DICPDMTO), and the Youth Promotion Association of the Chinese Academy of Sciences. (Text/Photo byChang Fei and Guo Jianping)

 Home
>>
Highlights
Home
>>
Highlights