Recently, a team led by Professor Chen Ping and Associate Professor Guo Jianping from our group has made significant advances in ammonia synthesis through chemical looping. Building on years of research in hydrogen storage materials, catalytic ammonia synthesis, and ammonia decomposition reactions, the team has developed a novel chemical looping method for ammonia synthesis utilizing hydrides and imino compounds. The relevant research results were published in Nature Energy (DOI: 10.1038/s41560-018-0268-z).
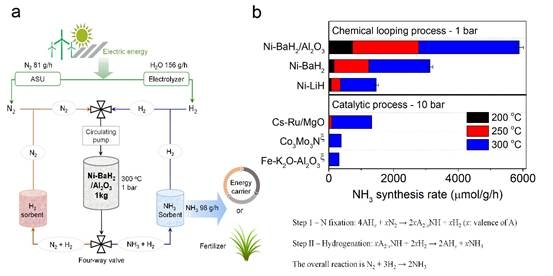
The synthesis of ammonia is related to food, water, air, and energy. New methods and processes for synthesizing ammonia based on renewable energy are cutting-edge topics in catalysis, materials, and energy chemistry. In catalytic reactions on transition metals, it is difficult to achieve low-temperature and efficient ammonia synthesis due to the inherent linear relationship (scaling relations) between the adsorption energies of the reactants. Decoupling the ammonia synthesis reaction into two sub-steps of nitridation and hydrogenation, i.e., the chemical chain process, is an effective strategy to circumvent the scaling relation.Building on previous findings, we introduced a low-temperature chemical looping ammonia synthesis technology that utilizes alkali (earth) metal imino compounds as nitrogen carriers. Initially, the hydrides of alkali (earth) metals produce the corresponding imino compounds by "fixing" nitrogen. Subsequently, the reaction environment changes to hydrogen, enabling the imino compounds to hydrogenate and yield ammonia.Among them,Li-NHandBa-NHsystems have moderate thermodynamics of nitridation and hydrogenation reactions. With the assistance of transition metal catalysts,ammonia synthesis can be achieved at atmospheric pressure and100°C. At 250 °C, the ammonia production rate of this process is about one order of magnitude higher than that of the highly activeCs-Ru/MgOcatalytic process. The chemical looping ammonia synthesis methods reported in the literature are mainly based onAl/AlN/Al2O3, Cr/CrN/Cr2O3,and other systems. Since they involve highly exothermic hydrolysisandendothermic reduction reactions, the temperatures required for these processes are very high(> 1000 ℃).
Based on the results of this study, we have constructed a chemical looping ammonia synthesis process based on renewable energy, that is, using electricity generated by renewable energy to produce nitrogen by air separation and hydrogen by electrolysis of water, and then alternately passing nitrogen and hydrogen into a reactor loaded with hydride for nitridation and hydrogenation reactions respectively, and the unreacted gas is separated and purified and recycled. This process can be carried out under relatively mild conditions (normal pressure,100-300 ℃), avoiding the high-energy gas compression process in theHaber-Boschprocess and significantly improving energy efficiency. Some units,such as synthetic ammonia and adsorption separation of ammonia,have been experimentally verified. This process provides a solution forstoring and utilizing renewable energy and constructingsmall-scale plantsforsynthetic ammonia distribution.
ProfessorChen Ping's research team has accumulated nearly 20 years of experience in the fields of alkali (earth) metal hydrides and (im)amino compounds.This research is another vital progress following the application of such compounds in hydrogen storage(Nature, 2002),catalytic ammonia decomposition(Angew. Chem. Int. Ed., 2015),and catalytic ammonia synthesis(Nature Chem., 2017).
This research was funded by the National Natural Science Foundation of China, the China-Japan Intergovernmental Cooperation Project, the Collaborative Innovation Center of Chemistry for Energy Materials of the Ministry of Education (iChEM ), our institute's Basic Research Project of New Technologies for Methanol Conversion and Coal-to-Oil Replacing (DICPDMTO), and the Youth Development Association of the Chinese Academy of Sciences.
(Text/Photos by Gao Wenbo and Guo Jianping)

 Home
>>
Highlights
Home
>>
Highlights