Reversible Hydrogen Uptake/Release over a Sodium Phenoxide–Cyclohexanolate Pair
Recently, a team led by Professor Chen Ping and Associate Professor He Teng from our group, in collaboration with Professor Wu Anan from Xiamen University, has made new progress in the research of hydrogen storage materials. The results of this work were published in Angewandte Chemie (Angew. Chem. Int. Ed.,Women in Chemistry of Angewandte Chemie), and this research paper was selected as the cover of the back page of the special issue (https://doi.org/10.1002/anie.201901616).

Hydrogen has been considered an ideal carrier for energy storage and transportation due to its high energy density and pollution-free properties. However, the technical bottleneck is the lack of safe and efficient hydrogen storage media. Therefore, developing cheap and efficient hydrogen storage and transportation carriers will provide an effective solution for using renewable energy. Liquid organic hydrogen storage materials are considered to be storage and transportation media with practical prospects because of their good stability, high hydrogen storage capacity, easy storage and transportation, and the ability to be built using existing oil and gas pipelines and gas stations. However, this type of material faces disadvantages such as unfavorable enthalpy change of dehydrogenation, high dehydrogenation temperatures, and reduced energy storage efficiency. Therefore, this type of material has not been put into practical use.
Recently, our research team proposed a new strategy: using the difference in electronegativity of metals to modify the electronic properties of organic hydrogen storage materials, and synthesized a novel organic-inorganic hybrid hydrogen storage system: metal organic compounds (MOCs). Theoretical calculation results show that the addition of metals can significantly reduce the dehydrogenation enthalpy of organic materials, and as the electron-donating properties of metals increase, the dehydrogenation enthalpy of materials decreases. In other words, by selecting different metals, the dehydrogenation enthalpy change of materials can be controllably adjusted, thereby thermodynamically controlling the dehydrogenation temperature of materials. This article takes sodium-modified phenol-cyclohexanol as an example, and its dehydrogenation enthalpy change has been reduced from 64.5 kJ/mol-H2 to 50.4 kJ/mol-H2. At the same time, it was found that as the electron-donating ability of the metal increases, the α-position C-H bond length of sodium cyclohexanol increases, and the two are in a linear relationship, which shows that the material has been activated after organic-inorganic hybridization, and the α-position C-H bond is preferentially broken during the dehydrogenation process.
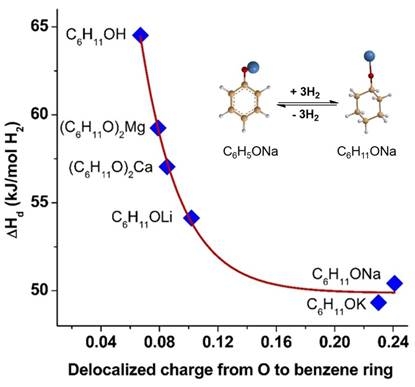
The experimental results show that the sodium phenolate-sodium cyclohexanol system can complete a reversible hydrogen storage cycle at 150 °C. After the material is dissolved in water for a hydrogen storage cycle reaction, the material's dehydrogenation temperature can be further reduced to below 100 °C. This is significantly lower than that of common liquid organic hydrogen storage materials. This article opens up new ideas for the future development of low-temperature reversible hydrogen storage materials.
The thermodynamic measurement part of this research work was completed in collaboration with Dr. Tom Autrey and Dr.Abhijeet Karkamkar of the Pacific Northwest National Laboratory in the United States. This work was funded by the National Natural Science Foundation of China, the key international cooperation project of the Ministry of Science and Technology, and the independent deployment fund of our institute. In addition, this is also one of the articles dedicated to the 70th anniversary of our institute. (Text/Photo by Yu Yang and He Teng).

 Home
>>
Highlights
Home
>>
Highlights