Recently, our group's Professor Chen Ping, associate Professor Liu Lin and Professor Li Hui from the Carbon Resource Small Molecules and Hydrogen Energy Utilization Innovation Zone Research Group (19T3 Group) have developed a high-performance finger-shaped and void-structured stainless steel palladium composite membrane that can meet the requirements of rapid start-up of hydrogen sources for fuel cells. The stainless-steel palladium composite membrane was used in an ammonia decomposition membrane reactor to produce hydrogen, and the temperature for complete conversion of ammonia decomposition was significantly reduced.
The metal palladium membrane separation used for hydrogen separation has the advantages of being small, silent, and compact. It is a key technology for fuel cell hydrogen sources. It can be combined with liquid solar fuels (such as methanol reforming or ammonia decomposition) to achieve "on-site production and use" of hydrogen. Liquid fuel hydrogen production coupled with palladium membrane purification technology can solve hydrogen's storage, transportation, and safety problems, and has broad application prospects in the fields of communication base station power supply, on-site hydrogen production at liquid solar hydrogenation stations, hydrogen-powered heavy trucks, and drones. Compared with ceramic palladium composite membranes, stainless steel palladium composite membranes have high mechanical strength and simple sealing advantages, which can meet the needs of small mobile applications.
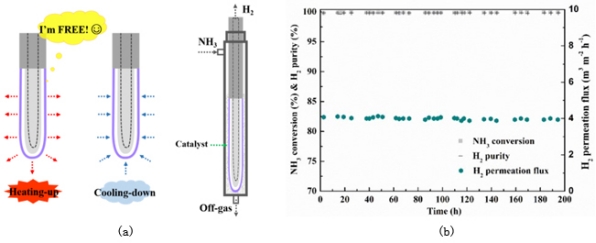
To address the challenge of developing a high-performance stainless steel palladium composite membrane, the research team first proposed a design featuring a finger-shaped and void structure. This design allows for the free expansion and contraction of the metal palladium membrane during rapid heating and cooling, thereby preventing damage to the membrane structure caused by contact between the stainless-steel substrate and the metal palladium membrane. The new stainless steel palladium composite membrane can achieve long-term stable operation for 2000 consecutive hours, and realize multiple rapid heating and cooling cycles under the conditions of simulating fuel cell use, meeting the requirements of rapid response of fuel cell hydrogen source. In addition, the void structure significantly reduces the permeation resistance of the porous carrier, the hydrogen permeation rate reaches 2.1E-6 mol/(m2*s*Pa), and the H2/N2 selectivity reaches 16000. The high-performance finger-shaped stainless steel palladium composite membrane is combined with the efficient ammonia decomposition catalyst Ru/MgO to form a membrane reactor, which can reduce the complete decomposition temperature of ammonia decomposition from more than 748 K in the literature to 673 K (ammonia decomposition conversion rate is 99.8%), and achieve 200 hours of continuous stable operation, indicating that the membrane reactor has certain potential for vehicle-mounted application.
Previously, in response to key issues in the industrial application of palladium composite membranes, the research team proposed a new route for in-situ defect repair, developed a number of new palladium composite membrane preparation technologies, and systematically studied the influence of synthesis gas composition on the hydrogen permeability of palladium membranes. They were invited to write two reviews (J. Mater. Chem.A, 2016, 4, 14069; and Chem. Eng. Sci., 2015, 127, 401). In 2019, the research team took the lead in building a large-scale (MW-scale) stainless steel palladium composite membrane production line at Zhangjiagang Industrial Research Institute, with a cost of only 1/10 of similar stainless steel palladium composite membranes abroad. At present, it has achieved a 10-kW system integration test of methanol reforming, palladium membrane purification, and hydrogen-oxygen fuel cells, as well as a hydrogen production technology demonstration (20 kg/d) of a liquid sunlight hydrogenation station.
The results of this work were published in full in the Journal of Chemical Engineering (Chem. Eng. J.). This work was supported by the Chinese Academy of Sciences (excellent project completion), the National Natural Science Foundation, the Ministry of Science and Technology's key field innovation team, and the Chinese Academy of Sciences' Lu Jiaxi International Team Project. (Text/Photo by Li Hui and Liu Lin)

 Home
>>
Highlights
Home
>>
Highlights