Recently, our research group, in collaboration with Professor Weidenthaler from the Max Planck Institute for Coal Chemistry in Germany and Associate Professor Wu Anan from Xiamen University, discovered a Ba-Cr quaternary nitride-hydride catalyst that achieves the catalytic synthesis of ammonia under relatively mild conditions.
Ammonia is not only the main raw material for nitrogen-based fertilizers, but also plays an important role as an energy carrier in the storage and conversion of renewable energy. The reaction conditions of the existing Haber-Bosch synthetic ammonia industrial process are very harsh (temperature 350 to 550 °C, pressure 10 to 30 MPa). The development of low-temperature, low-pressure,and highly active catalysts has been a long-term goal pursued by researchers across the world. At present, most researchers develop catalysts based on active metals such as iron and ruthenium. In comparison, there is relatively little research on pre-transition metals such as chromium.
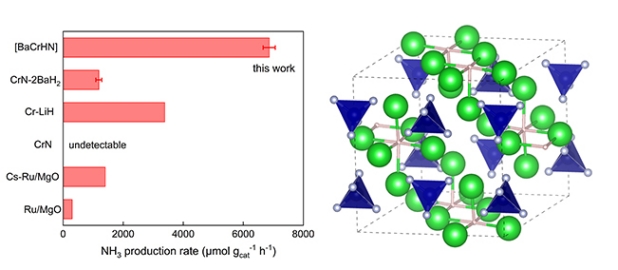
In this work, based on previous work (Angew. Chem. Int. Ed., 2015; Nat. Chem., 2017; J. Am. Chem. Soc., 2018), our group reported a new strategy for the design of ammonia synthesis catalysts, namely, preparing barium-chromium quaternary nitrogen hydride catalysts from barium and chromium amide compounds, with the catalyst active phase being Ba5CrN4H. This unique structure can give lattice nitrogen and latticehydride ion(H-) higher reactivity, and can achieve effective ammonia synthesis catalysis under mild conditions. For example, under 573 K and 10 bar conditions, the ammonia synthesis rate of this catalyst is 6.8 mmol NH3 cat-1h-1, which is more than 3 orders of magnitude higher than that of chromium nitride catalysts and about 4 times that of Cs-Ru/MgO catalysts. This research work further enriches the understanding of "the role of hydrides in ammonia synthesis catalysis", and also provides new ideas for the development of new ammonia synthesis catalysts, especially for the design of pre-transition metal catalysts that are generally considered to have low activity.
These findings were published in the Cell Press journal Chem Catalysis under the title "Barium Chromium Nitride-hydride for Ammonia Synthesis" and were selected as the cover article. The first authors of this work are our group's doctoral student Guan Yeqin and postdoctoral fellow Zhang Weijin. The work was supported by the National Natural Science Foundation of China, the Chinese Academy of Sciences Youth Innovation Promotion Association, and other projects. (Text/Photo by Guan Yeqin).
Article link: https://doi.org/10.1016/j.checat.2021.08.006

 Home
>>
Highlights
Home
>>
Highlights