Recently, a research team lead by Professor Chen Ping and Professor Guo Jianping from our group, in collaboration with a team of Professor Tejs Vegge from the Technical University of Denmark, a team of Li Haiyang and a team of Jiang Ling from our institute, have made important progress in the research of catalytic synthesis of ammonia. For the first time, the team applied coordinated hydride materials to the catalytic synthesis of ammonia, then developed a new type of alkaline (earth) metal ruthenium-based ternary hydride catalyst, and realized the catalytic synthesis of ammonia under mild conditions.
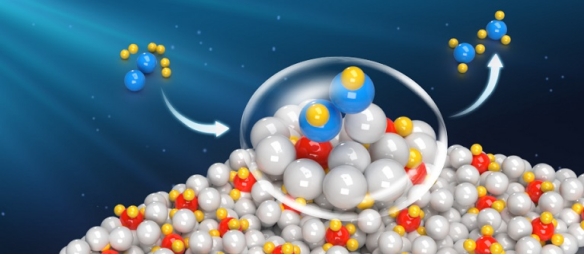
Ammonia is an important chemical raw material and a promising energy carrier. The realization of efficient synthesis of ammonia under mild conditions has important scientific significance and practical value. The existing synthetic ammonia industry driven by fossil energy is a high-energy consumption and high-carbon emission process. Therefore, in the "green" synthetic ammonia process driven by renewable energy, the development of low-temperature, low-pressure and high-efficiency synthetic ammonia catalysts is the core technology and the goal that scientific Researchers have been pursuing for a long time.
In this work, the alkali (earth) metal ruthenium-based ternary hydride (Li4RuH6 and Ba2RuH6) catalyst materials developed by the team could realize the catalytic synthesis of ammonia under mild conditions. The catalyst material is an ionic compound composed of Ru's coordinated anion [RuH6]4-and alkali (earth) metal cations Li+or Ba2+, which has excellent catalytic synthesis of ammonia at low temperature (<573 K) and low pressure (<10 bar). When the reaction temperature is as low as 100 oC, the Ba2RuH6 catalyst still has detectable catalytic activity. The study found that the ammonia synthesis reaction of this type of ternary hydride catalyst material follows a hydrogen-assisted dissociation mechanism, and all its components participate in the ammonia synthesis reaction, that is, the electron-rich [RuH6]4- is the N2 activation site, H- is the electron and proton transfer carrier, Li+or Ba2+ reduces the reaction energy barrier by stabilizing the NxHy species, and through multi-component synergistic catalysis, N2 and H2 are converted into NH3 through an energy-optimal reaction path.
As a unique type of compound catalyst, this type of ternary hydride catalyst is significantly different from conventional heterogeneous ammonia synthesis catalysts in terms of composition, structure, reaction kinetics, and the mechanism of action of active centers. It is related to homogeneous ammonia synthesis catalysts and has built a bridge between heterogeneous nitrogen fixation and homogeneous nitrogen fixation research. More importantly, this study enriched the ammonia synthesis catalyst system and proposed a design strategy for ammonia synthesis catalysts to construct "electron-rich, multi-component active sites", providing new ideas for further exploring low-temperature, low-pressure, and efficient ammonia synthesis catalysts.
Professor Chen Ping's team has accumulated more than 20 years of experience in hydride material research. This research is an other important progress following the application of hydrides in hydrogen storage (Nature, 2002), catalytic ammonia synthesis (Nat. Chem., 2017), and chemical looping ammonia synthesis (Nat. Energy, 2018).
The related results were recently published in Nature Catalysis under the title of "Ternary Ruthenium Complex Hydrides for Ammonia Synthesis via the Associative Mechanism" . The first authors of this work are Wang Qianru, a DNL1901 doctoral student of our institute, and Dr. Jaysree Pan of the Technical University of Denmark. The above work was supported by the Basic Science Center Project "Air Main Component Conversion Chemistry" of the National Natural Science Foundation of China and the Youth Innovation Promotion Association of the Chinese Academy of Sciences. (Text/Photo by Wang Qianru and Jaysree Pan)
Article link: https://doi.org/10.1038/s41929-021-00698-8

 Home
>>
Highlights
Home
>>
Highlights