Recently, a research team led by Professor Chen Ping and Professor Guo Jianping in our group, in collaboration with a team of Professor Tejs Vegge from the Technical University of Denmark, revealed the reaction mechanism of nitrogen fixation and hydrogenation to produce ammonia by non-transition metal-based barium hydride (BaH2) through a combination of experimental design and theoretical calculations.
Ammonia is one of the basic chemical raw materials and is the nitrogen source for synthetic nitrogen fertilizers and almost all important nitrogen-containing chemicals. In addition, ammonia can also be used as an energy carrier or fuel, playing a vital role in human life. The reaction conditions of the traditional industrial ammonia synthesis process are very harsh. In-depth research on the activation and conversion mechanism of nitrogen and the development of new nitrogen fixation and ammonia synthesis processes are the unremitting goals of researchers. It is generally believed that transition metals are key components of heterogeneous and homogeneous catalysts for ammonia synthesis and the active center of nitrogenase. Because the main group elements have different bonding and reaction characteristics, their nitrogen fixation reaction chemistry has gradually attracted the attention of researchers in recent years.
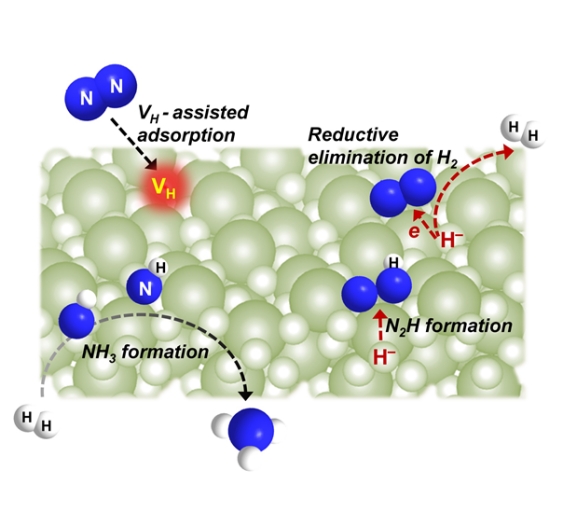
Based on the previous work of our research group (Nat. Chem. 2017; Nat. Energy 2018; Nat. Catal.,2021), this work took the well-structured alkaline earth metal hydride BaH2 as the compound of interest, then combined experimental characterization and theoretical calculation results, and found that the existence of hydrogen vacancies on the surface of BaH2 plays a key role in the adsorption and activation of nitrogen. On the one hand, the hydride ions in BaH2 undergo a reduction-elimination reaction to release hydrogen and transfer electrons to barium atoms, which helps to further weaken the nitrogen-nitrogen bond. On the other hand, the negative hydrogen species can undergo a reductive protonation reaction to form a nitrogen-hydrogen bond. This is similar to the nitrogen fixation process of nitrogenase and certain molecular hydride complexes. This work clarifies the nitrogen fixation mechanism of alkaline (earth) metal hydrides and provides new ideas for the further design of non-transition metal-based materials for efficient nitrogen fixation and ammonia synthesis.
This research, titled "Transition-Metal-Free Barium Hydride Mediates Dinitrogen Fixation and Ammonia Synthesis", was recently published in Angew. Chem. Int. Ed., with the first author being Guan Yeqin, a doctoral student inour research group. The work was supported by projects, such as the National Natural Science Foundation of China and the Youth Innovation Promotion Association of the Chinese Academy of Sciences. (Text/Photo by Guan Yeqin)
Article link: https://doi.org/10.1002/anie.202205805

 Home
>>
Highlights
Home
>>
Highlights