Recently, our research group collaborated with a team of Associate Professor Wu Anan from Xiamen University and found that alkaline (earth) metal hydrides, such as lithium hydride (LiH), can mediate the hydrogenolysis of aniline C-N bonds to generate benzene and ammonia (abbreviated as CL-HDN) through a chemical looping, in which the nucleophilic attack of hydride ions (Hˉ) on the benzene ring to promote the cleavage of the aromatic C-N bond is a key step in the process.
The activation and cleavage of C-N bondsin nitrogen-containing organic compoundsis a very important chemical reaction process, which has been widely studied in the fields of multiphase, homogeneous, and enzyme chemistry. Among them, the sp2 C-N bond energy in aromatic amine compounds is high, the reaction activity is low, and the amine group has poor leaving ability. It is a very challenging research topic to achieve direct cleavage of sp2 C-N bonds under mild conditions.
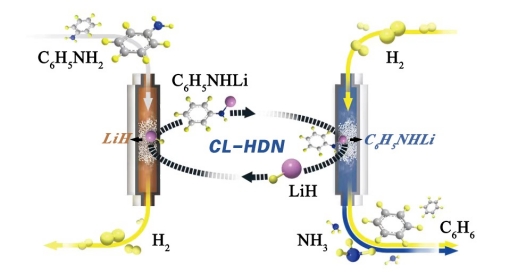
Based on previous research on hydride-mediated ammonia synthesis (Nat. Chem., 2017; Nat. Energy, 2018) and metal-organic hydride hydrogen storage (Angew. Chem. Int. Ed., 2019), the research team proposed a chemical looping process for LiH-mediated hydrogenolysis of aniline C-N bonds, consisting of three steps:
1) LiH reacts with aniline to form lithium aniline (C6H5NHLi),
2) Lithium aniline undergoes hydrogenolysis in a hydrogen atmosphere to produce products benzene and lithium amide (LiNH2),
3) LiNH2 is hydrogenated to release ammonia and regenerate LiH.
In this process, benzene is the only organic product of denitrification, while on conventional transition metal catalysts, the saturated hydrogenation product of the benzene ring is usually the main product; at the same time, at lower reaction temperature and pressure, the benzene generation rate is comparable to that of transition metal catalytic reactions. Combined with theoretical calculations, the research team found that H2 can be activated by heterolytic cleavage of the Li-N bond of lithium aniline to form a [LiH-aniline] complex, and the hydride ion (Hˉ) bound to Li+ acts as a nucleophile to attack the positively charged α- C atom on the benzene ring. At the same time, Li+ forms a cation-π interaction with the benzene ring to weaken the C-N bond, thereby achieving the sp2 C-N cleavage to generate benzene. This study provides new ideas for the study of C-N bond activation of nitrogen-containing organic matter. More importantly, the discovery of the mechanism for nucleophilic substitution of the benzene ring mediated bythe hydride ion (H-) may inspire further research into related chemical transformations.
This work was recently published in the Journal of the American Chemical Society under the title of "Transition Metal-Free Hydrogenolysis of Anilines to Arenes Mediated by Lithium Hydride." The co-first authors of this work are Cai Yongli, a doctoral student in our group, and Liu Wei, a graduate student at Xiamen University. This work was funded by the National Natural Science Foundation, the Youth Innovation Promotion Association of the Chinese Academy of Sciences, the Liaoning Province "Xingliao Talent Plan,"and other projects. (Text/Photo byCai Yongli).
Article link: https://pubs.acs.org/doi/full/10.1021/jacs.2c05586

 Home
>>
Highlights
Home
>>
Highlights