Recently, a team led by Professor Chen Ping and Professor Guo Jianping in our group has made new progress in hydride chemical nitrogen fixation research. They have revealed the relationship between the photodehydrogenation color change phenomenon of lithium hydride (LiH) and nitrogen fixation, thus constructing a LiH-mediated photocatalytic ammonia synthesis process.
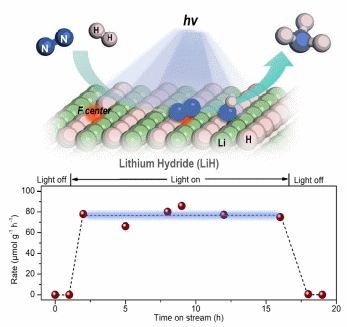
The synthesis of ammonia through the hydrogenation of nitrogen is a key chemical reaction essential for sustaining life on Earth and fulfilling the energy and chemical industry demands of human society. However, the existing Haber-Bosch ammonia synthesis technology necessitates harsh reaction conditions of high temperature and pressure (>400 ºC, >100 bar), resulting in a high energy consumption and carbon emissions. Developing novel ammonia synthesis methods driven by renewable energy and operated under milder conditions has been a long-term goal for researchers and presents a significant challenge in the field of chemical science. Solar energy stands as an inexhaustible renewable resource, making the photo-powered ammonia synthesis process one of the most promising approaches to ammonia production.
Based on previous studies (Nat. Chem., 2017; Nat. Energy, 2018; Nat. Catal., 2021), this study explores the nitrogen fixation behaviour of hydrides under light. The research team found that LiH, as an inorganic wide bandgap semiconductor, dehydrogenates and changes colour under ultraviolet light. Unlike conventional oxide or nitride semiconductors, after LiH produces carrier separation, hydride ions (H-) lose electrons to form H2 and generate hydrogen vacancies. Meanwhile, photogenerated electrons can create electron-rich colour central structures at the surface hydrogen vacancies, facilitating the reduction and activation of nitrogen. In this process, hydride ions also participate in the formation of NH bonds. Under conditions of nitrogen and hydrogen co-feeding, the research team achieved the LiH-mediated photocatalytic synthesis of ammonia under mild conditions. This work demonstrates the development potential of hydrides in mediating photochemical reactions and enriches the knowledge system of hydride-based nitrogen fixation chemistry.
The results of this work, titled "Light-driven ammonia synthesis under mild conditions using lithium hydride", were recently published in Nature Chemistry. The first authors of the work are doctoral student Guan Yeqin and postdoctoral fellow Wen Hong. This work was supported by the National Key R&D Program, the National Natural Science Foundation, the Chinese Academy of Sciences Youth Innovation Promotion Association, and other projects. (Text / Photo by Guan Yeqin)
Article link: https://doi.org/10.1038/s41557-023-01395-8

 Home
>>
Highlights
Home
>>
Highlights